1 Chloro 4 Nitrobenzene Solid Derivative: A Comprehensive Overview
1-chloro-4-nitrobenzene, a compound with a rich history and diverse applications, has been a subject of considerable interest in the scientific community. This article delves into the details of this solid derivative, exploring its chemical properties, synthesis methods, and potential uses.
Chemical Properties
1-chloro-4-nitrobenzene, with the chemical formula C6H3ClNO4, is a solid derivative of nitrobenzene. It is characterized by a yellowish-brown color and a melting point of approximately 114掳C. The compound is highly soluble in organic solvents such as acetone, chloroform, and benzene, but has limited solubility in water.
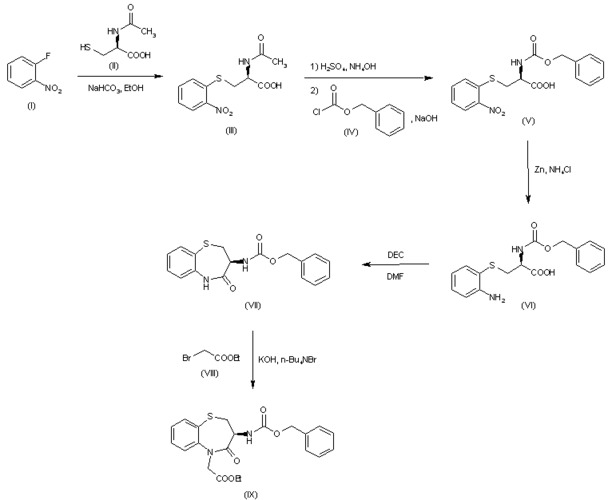
One of the key features of 1-chloro-4-nitrobenzene is its strong nitro group (-NO2), which imparts a high degree of reactivity to the compound. This reactivity is evident in its ability to undergo various chemical transformations, such as nucleophilic substitution, electrophilic aromatic substitution, and reduction reactions.
Synthesis Methods
There are several methods for synthesizing 1-chloro-4-nitrobenzene, each with its own advantages and limitations. The most common methods include:
-
Direct chlorination of 4-nitrobenzene: This method involves the direct chlorination of 4-nitrobenzene using a chlorinating agent such as chlorine gas or phosphorus pentachloride. The reaction is typically carried out in the presence of a catalyst, such as ferric chloride or aluminum chloride.
-
Reaction of 4-nitrobenzene with thionyl chloride: This method involves the reaction of 4-nitrobenzene with thionyl chloride (SOCl2) to form 4-nitrobenzenesulfonyl chloride, which is then hydrolyzed to yield 1-chloro-4-nitrobenzene.
-
Reduction of 4-nitrochlorobenzene: This method involves the reduction of 4-nitrochlorobenzene using a reducing agent such as sodium borohydride or lithium aluminum hydride.
Applications
1-chloro-4-nitrobenzene finds applications in various fields, including:
-
Pharmaceuticals: The compound is used as a starting material for the synthesis of various pharmaceuticals, including anti-inflammatory drugs, analgesics, and antiviral agents.
-
Agrochemicals: 1-chloro-4-nitrobenzene is used in the synthesis of agrochemicals, such as herbicides and insecticides.
-
Textiles: The compound is used in the dyeing and finishing of textiles, providing resistance to fading and abrasion.
-
Polymers: 1-chloro-4-nitrobenzene is used in the synthesis of polymers, such as polychlorinated biphenyls (PCBs) and polychlorinated naphthalenes (PCNs).
Environmental Impact
Like many organic compounds, 1-chloro-4-nitrobenzene can have adverse effects on the environment. The compound is highly toxic to aquatic organisms and can accumulate in the food chain. It is also classified as a persistent organic pollutant (POP), meaning it can persist in the environment for a long time and travel long distances through air and water.
Efforts are being made to minimize the environmental impact of 1-chloro-4-nitrobenzene and other similar compounds. This includes the development of safer alternatives and the implementation of proper waste management practices.
Conclusion
1-chloro-4-nitrobenzene is a versatile solid derivative with a wide range of applications. Its unique chemical properties and reactivity make it a valuable compound in various industries. However, its environmental impact cannot be overlooked, and efforts must be made to mitigate its adverse effects.
| Method | Reaction Conditions | Yield |
|---|---|---|
Direct chlorination
相关文章 |
