1,3-Nitrophenyl Ethanol: A Detailed Overview
1,3-Nitrophenyl ethanol, a compound with a rich history in organic chemistry, has garnered significant attention for its unique properties and applications. In this article, we delve into the intricacies of this compound, exploring its structure, synthesis, properties, and uses.
Structure and Formula
1,3-Nitrophenyl ethanol is an organic compound with the chemical formula C8H7NO4. It consists of a phenyl group (C6H5) attached to a nitro group (NO2) at the 1-position and an ethanol group (CH2OH) at the 3-position. The molecular structure of 1,3-nitrophenyl ethanol is as follows:
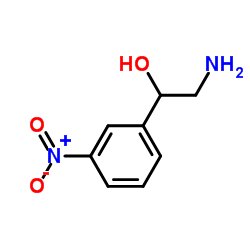
| Atom | Symbol | Number |
|---|---|---|
| Carbon | C | 8 |
| Hydrogen | H | 7 |
| Nitrogen | N | 1 |
| Oxygen | O | 4 |
The presence of the nitro group imparts a yellowish color to the compound, while the ethanol group contributes to its solubility in organic solvents.
Synthesis
1,3-Nitrophenyl ethanol can be synthesized through various methods, including the nitration of phenol followed by the reaction with ethanol. One common synthesis involves the following steps:
- Nitration of phenol: Phenol is nitrated using a mixture of concentrated nitric acid and concentrated sulfuric acid to form 2-nitrophenol.
- Reduction of 2-nitrophenol: The 2-nitrophenol is then reduced to 1-nitrophenol using a reducing agent, such as sodium borohydride (NaBH4) or tin(II) chloride (SnCl2).
- Reaction with ethanol: Finally, 1-nitrophenol is reacted with ethanol in the presence of an acid catalyst, such as hydrochloric acid (HCl), to yield 1,3-nitrophenyl ethanol.
This synthesis method is relatively straightforward and yields a high purity of the desired compound.
Properties
1,3-Nitrophenyl ethanol exhibits several distinct properties that make it valuable in various applications:

- Color: As mentioned earlier, the compound has a yellowish color due to the presence of the nitro group.
- Solubility: 1,3-Nitrophenyl ethanol is soluble in organic solvents such as ethanol, acetone, and chloroform, but it is only slightly soluble in water.
- Odor: The compound has a characteristic, somewhat unpleasant odor.
- Boiling Point: The boiling point of 1,3-nitrophenyl ethanol is approximately 243掳C.
- Melting Point: The melting point of the compound is around 57掳C.
Applications
1,3-Nitrophenyl ethanol finds applications in various fields, including:
- Pharmaceuticals: The compound is used as an intermediate in the synthesis of certain pharmaceuticals, such as antiviral drugs.
- Agrochemicals: It is employed in the production of agrochemicals, including herbicides and insecticides.
- Textiles: 1,3-Nitrophenyl ethanol is used in the dyeing and finishing of textiles.
- Research: The compound is a valuable reagent in organic synthesis and serves as a substrate for various chemical transformations.
Its unique properties make it an essential component in numerous research and industrial applications.
